
 |
Immunofluoresence
The staff are called over to a microscope to look at immunofluoresence. We are asked, "Look at the bright red dots; are they real?" or "See the green lining on the tubules; is this real?"
We reply, "Let's compare these slides to your controls." The investigator looks back at us with a squinched up face puzzled expression. So we repeat, "Let's look at the controls."
"Oh, but we didn't do the controls this time. We just wanted to do it fast to see if we got any results," is a common response.
But without controls, how does anybody know what is real, true and accurate from what is artefact?
Troubleshooting nonspecific labelingIn the following example, there are two probes. Each one is an antibody probe.
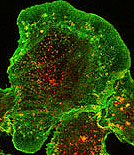 |
This is the perfect experiment. All antibodies are binding
specifically.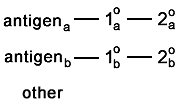 |
Here are some common, and not so common, problems that occur with staining and controls to test for them..
| The problem | The control(s) | |
|
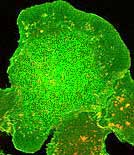
Here is one of the most common problems. One or more of
the antibodies stick to stuff that has nothing to do with the experiment. In this example,
one of the secondary antibodies is causing a huge amount of background. 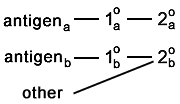 And in this example, one of the secondaries is binding to an antigen. This appears as colocalization. 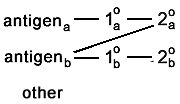 |
These two controls check to make sure that no secondaries are
binding nonspecifically. In each case, the sample should appear completely
unstained.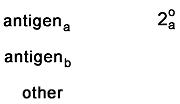
|
 |
Here is a problem which is more common than you'd think.
One (or both) of the secondaries binds to the wrong primary. This appears as
colocalization.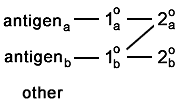 |
These two controls check for crosstalk from one secondary to
the other's primary. In each case, the sample should appear completely unstained.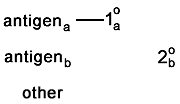
|
 |
Here is a rare problem. The secondaries bind to each
other.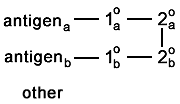 |
This control makes sure that the secondaries do not bind to
each other. In this case, the sample should appear compleely unstained.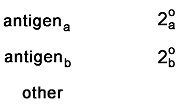 |
 |
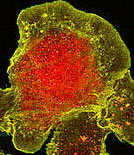
And, of course, there is the problem of autofluorescence.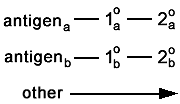 |
The control is simple; don't stain with anything. But
bring the sample through the same fixation and washes. In this case, the sample should
appear compleely unstained. |
|
One (or both) of the primary antibodies sticks to something
it is not supposed to. You caught this if the whole background lights up.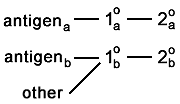 But if it binds selectively to the other antigen, it is going to appear as colocalization. 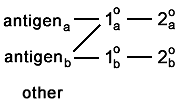 And if it binds to something else that is sparse or very specifically located, then you're not going to catch the artefact. 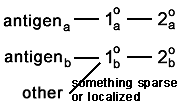 |
What does the Western blot (of whole lysate) look like? Or selective KOs? |
last revised 19 November 2001 by mc