| The Services: Transmission Electron
Microscopy
 JEOL
1200EX This instrument offers the highest basic performance as a 120
kv transmission electron microscope employing a uniquely designed 3-stage 6-lens imaging
system. It offers operational ease, excellent image quality and high resolution at
low to high magnifications. It is equipped with side entry goniometer stage, minimum dose
system, bottom mounted high resolution Gatan video camera and side mounted wide angle
Gatan video camera. JEOL
1200EX This instrument offers the highest basic performance as a 120
kv transmission electron microscope employing a uniquely designed 3-stage 6-lens imaging
system. It offers operational ease, excellent image quality and high resolution at
low to high magnifications. It is equipped with side entry goniometer stage, minimum dose
system, bottom mounted high resolution Gatan video camera and side mounted wide angle
Gatan video camera.
JEOL 100CXII This high
performance 100 kv transmission electron microscope features a cool beam electron gun,
high image contrast, high-speed cascade differential evacuation, optimum underfocus system
using an image wobbler and a side entry goniometer.
Cryo Transmission Electron Microscopy for single molecule
imaging.
The College, HHMI and NIH (by way of an awarded Shared Instrumentation Grant) have
supported establishing a full Cryo EM program. The technology spans the resolution
range from electron microscopy to X-ray crystalography and allows for imaging of single
molecules in their hydrated state. Please read more about the technology.
Scanning Electron Microscopy
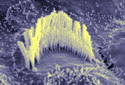 JEOL 6400 This high
performance Scanning Electron Microscope operates with accelerating voltage from 0.2 kv to
35 kv utilizing a high brightness LaB6 filament. It
offers full keyboard operation, framestore with digital image processing and digital image
capture on a PC running analySIS software. JEOL 6400 This high
performance Scanning Electron Microscope operates with accelerating voltage from 0.2 kv to
35 kv utilizing a high brightness LaB6 filament. It
offers full keyboard operation, framestore with digital image processing and digital image
capture on a PC running analySIS software.
Specimen Preparation for Electron Microscopy
The staff of the AIF offer full service sample
preparation for many standard and state of the art EM techniques. These include:
- E
 mbedding utilizing either epoxy or acrylic
resins at ambient or low temperatures. mbedding utilizing either epoxy or acrylic
resins at ambient or low temperatures.
- Thin Sectioning with a Reichert Ultracut E or
Leica UCT ultramicrotome.
- Negative Staining.
- Freeze Fracture using a Cressington CFE-50
Freeze Etch Unit.
- Immunogold Labeling following pre or post
embedding protocols.
- Critical Point Drying with a Tousimis Samdri
790 Critical Point Dryer and Sputter Coating using a Denton Sputter Coater for
preparing cells and tissues for SEM Imaging.
- Cryoultramicrotomy
utilizing a Leica UCT cryoultramicrotome for optimizing epitope availability and morphological preservation for immunogold
labeling.
 Slam Freeze
Cryofixation using a Life Cell CF100 Slam Freezer, which can be followed by High
Resolution Rotary Shadowing in a Cressington CFE-50 equipped with e-beam guns for
platinum or tungsten-tantalum evaporation. Slam Freezing followed by rotary shadowing is a
powerful technique to obtain high resolution images of cells or macromolecules in 3-D,
that have been frozen in the hydrated state. Slam Freeze
Cryofixation using a Life Cell CF100 Slam Freezer, which can be followed by High
Resolution Rotary Shadowing in a Cressington CFE-50 equipped with e-beam guns for
platinum or tungsten-tantalum evaporation. Slam Freezing followed by rotary shadowing is a
powerful technique to obtain high resolution images of cells or macromolecules in 3-D,
that have been frozen in the hydrated state.- Slam Freeze Cryofixation using a Life Cell
CF100 Slam Freezer followed by Freeze Substitution and Low Temperature Embedding
in a Bal Tec FSU-010 Freeze Substitution Unit. Freeze Substitution is an alternative
method for optimizing epitope preservation for immunogold labeling.
Photographic
Documentation for Electron Microscopy
The AIF offers a photographic service
for producing high quality electron micrographs on conventional photographic paper or high
resolution scanning and direct digital printing for poster, lecture, web and journal
publication.
Routine
light microscopy
A Zeiss AxioSkop II with optics for brightfield, darkfield (through the condenser or via
true oblique illumination), phase contrast, Nomarski, polarized light and epi-fluorescence
with1.25X through 100X objectives serves as the "routine" microscope.
Images are recorded with a color Zeiss AxioCam.
To meet the growing demand for imaging of live material, epecially of eGFP or other
fluorescently tagged cells, or of cells in culture dishes, the AIF has Olympus inverted
microscopes with a wide array of optics and photography options. On other inverted
systems in the AIF researchers continue to use video digitizing technology for imaging
motile cells in phase contrast.
Stereo Dissection
Microscope
Lower magnification imaging is
achieved with a Zeiss SV11 ("STEMI") with a Retiga 1300 digital camera, optical
tunable filter for color imaging and IP Lab for image capture. Reflected light is
provided either by a ring illuminator or by two point illumination, transmitted light is
provided with a continuously adjustable 100% transmittance to darkfield slider and
epi-illumination for fluorescence is provided by a mercury arc lamp with filters for dapi,
CFP, GFP, YFP or rhodamine/RFP. [More
info and instructions.]
BioRad Radiance
2000 Laser Scanning Confocal Microscope
Thin optical sections of much higher resolution than
normal epi-fluorescence can be obtained from live or fixed cultured cells, vibratome sections, or intact tissue with
colocalization of up to three different
fluorescent probes and one reflectant probe. Collected images can be reconstructed in 3D, enhanced, or analyzed using a
variety of techniques. Data can be readily ported to other platforms for analysis or for
final presentation. [Instructions for use.]
Leica SP2 AOBS Laser Scanning
Confocal Microscope
True spectral imaging with thin optical sections of
much higher resolution than normal epi-fluorescence can be obtained from cultured cells,
vibratome sections, or intact tissue with simultaneous colocalization of multiple
fluorescent probes, reflectance and transmitted light. Collected images can be
reconstructed in 3D, enhanced, or analyzed using a variety of techniques. Data can be
readily ported to other platforms for analysis or for final presentation. This
instrument also has powerful capabilities for FRAP, FRET and time
lapse applications. [More info and
instructions.]
Leica SP5 AOBS Laser Scanning
Confocal Microscope
Newer model of SP2. Arriving late December 2007.
For installation in new satellite facility on second floor of Michael F. Price Center for Genetic and Translational Medicine
in the Harold and Muriel Block Research Pavilion in January/February 2008.
Zeiss Live/DUO confocal microscope
High speed confocal microscope designed specifically for photoactivation or bleaching
via a separate scanner than the imaging path. This confocal with a 100 mW laser at
489 nm and 50 mW lasers at 405 and 561 nm will be used primarily for live cell
applications. [More info and
instructions.]
PerkinElmer UltraVIEW RS-3 Spinning Disk Laser
Confocal Microscope
Preferable for imaging live cell cultures due to reduced phototoxcity, thin optical
sections may be imaged as time lapse volumes. The 9 fps full field 12 bits imaging
system has laser lines at 488, 568 and 647 nm for exciting three popular ranges of
fluorescent probes, a piezo for high speed and reproducable Z axis control, environmental
control and Nikon optics. Expected move to new satellite facility on second floor of Michael F. Price Center for Genetic and
Translational Medicine in the Harold and Muriel Block Research Pavilion in
January/February 2008.
Multi Photon
Confocal Microscopy
Multi-photon microscopy relies on excitation of fluorophores or harmonic
generation by femptosecond pulses of highly concentrated long wavelength light.
Practically, this allows for imaging multiple fluorescent wavelengths deep in live
tissue. The system reduces bleaching and other problems endemic to epi-fluorescent
microscopy, may be more sensitive due to its lack of a confocal pinhole, and solves other
problems of light scatter. The instrument is at the center of the intravital
imaging program at AECOM. [Instructions for use.]
FRET
Fluorescence Resonance Energy Transfer, the transfer of energy from a donor fluorophore
within 7 nm of an acceptor fluorophore, can be used to measure binding interactions
between and within molecules. The AIF provides acceptor bleaching for FRET imaging
and measurements on three confocal microscopes and ratio FRET with widefield microscopy.
Widefield FLIM in the time domain using a gated cooled CCD camera
with LaVision software may be available.
TIRF
Total Internal Reflection Fluorescent microscopy provides excitation of
fluorophores only within 100 nm of the subtrate. Therefore, only molecules
immediately apposed to the coverslip are excited and imaged. Objective illuminated
TIRF is provided on an Olympus IX71 with either a 60X or 100X N.A. 1.45 or a 100X N.A.
1.65 with capability to do TIRF/FRET using probes over the visible spectrum from CFP
through red. Image collection and automated shuttering are provided with an Andor EM
camera and Uniblitz shutters running under IPLab.
D.I.C. (Nomarski),
Darkfield, Phase Contrast, IRM, and Epifluorescence with digital imaging
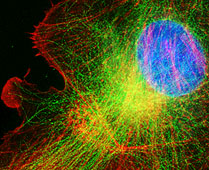 Four inverted microscope
stations for high spatial resolution, wide dynamic range (low light to bright light) with time lapse and deconvolution capabilities. 12 bit
Cooke Sensicam QE cooled CCD cameras mounted on high efficiency throughput Olympus IX70 or
IX81 inverted microscopes with state of the art infinity corrected optics. May be used to
collect multiple fluorescent probes and transmitted light (brightfield, phase contrast or
Nomarski) images with IPLab software running on PCs. Environmental chambers for the
Olympus microscopes are available for in vivo work. Many applications including
spot photometry. Also, focus motors for collection of serial sections for deconvolution.
Deconvolution produces images that are confocal-like in their resolution but may have a
benefit of imaging weak signal or a wide dynamic range. Standard fluorescent filters
include FITC, rhodamine, Cy3, Cy5, Dapi, GFP, CFP, YFP among others more esoteric ones and
a 50/50 mirror for IRM.
[Instructions for use.] Four inverted microscope
stations for high spatial resolution, wide dynamic range (low light to bright light) with time lapse and deconvolution capabilities. 12 bit
Cooke Sensicam QE cooled CCD cameras mounted on high efficiency throughput Olympus IX70 or
IX81 inverted microscopes with state of the art infinity corrected optics. May be used to
collect multiple fluorescent probes and transmitted light (brightfield, phase contrast or
Nomarski) images with IPLab software running on PCs. Environmental chambers for the
Olympus microscopes are available for in vivo work. Many applications including
spot photometry. Also, focus motors for collection of serial sections for deconvolution.
Deconvolution produces images that are confocal-like in their resolution but may have a
benefit of imaging weak signal or a wide dynamic range. Standard fluorescent filters
include FITC, rhodamine, Cy3, Cy5, Dapi, GFP, CFP, YFP among others more esoteric ones and
a 50/50 mirror for IRM.
[Instructions for use.]
Exhaustive Photon
Reassignment (EPR)
EPR deconvolution complements the
other deconvolution techniques offered at the AIF by providing preservation of the total
energy of the sampled volume for quantitative analysis of very dim specimens.
Automated imaging of multiply probed serial optical sections is performed with a piezo
controller and a Photometrics 15 bit cooled CCD camera on a high efficiency upright
Olympus microscope. This is a specialty technique to image single or few
molecules with precise locating within 70 nm.
Microinjection
Microinjection is a method to deliver
solutions (proteins, DNA or RNA, other chemicals) directly into individual cells in
culture. The AIF has two automated Eppendorf systems for use on any inverted
microscopes in the Facility including the confocal, multirphoton, and other digital
imaging systems. [A movie of cells
being injected.] [Instructions
for use.]
Motion Analysis
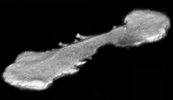 High speed (200 FPS under bright
illumination), real time (30 FPS video), or time lapse (approx. 100 ms to hours) imaging
with fluorescence and transmitted light can be performed on inverted microscopes with
temperature regulated environmental chambers. Images can be made into movies for
video or web presentations. Sophisticated morphometric measurements may be made over
time. Quantification of images includes intensity changes of fluorescence, changes
in cell or particle velocity, direction, shape and size over time and schematic
visualization of such changes. In some cases, volume changes can be measured. High speed (200 FPS under bright
illumination), real time (30 FPS video), or time lapse (approx. 100 ms to hours) imaging
with fluorescence and transmitted light can be performed on inverted microscopes with
temperature regulated environmental chambers. Images can be made into movies for
video or web presentations. Sophisticated morphometric measurements may be made over
time. Quantification of images includes intensity changes of fluorescence, changes
in cell or particle velocity, direction, shape and size over time and schematic
visualization of such changes. In some cases, volume changes can be measured.
Volume Rendering and
3D Quantitation
For 3D rendering or reconstructions the staff operate and train Imaris Bitplane,
Voxx, a number of plugins within ImageJ and Volocity. The staff train investigators
in more simple 3D imaging and quantitation via analysis of serial sections with ImageJ and
I.P. Lab including the authoring of scripts for automation and result reporting.
Single Photon Uncaging
Uncaging is the activation by removing a photo-labile blocking group from DNA,
RNA, protein or small molecules. The uncaging station consists of an Olympus IX70,
two Hg arc lamps for UV uncaging and epifluorescence, UV corrected and phase contrast
optics for uncaging and viewing cell behavior, shutters for high speed and timed uncaging
and image collection, and a Cooke Sensicam for recording uncaged fluorescence. This
system shares a microscope with a microinjection apparatus
for ease of loading cells for live experiments. A 337 nm laser with has been
purchased for the system and is under development on a separate microscope stand in the
Biophotonics Innovation Laboratory.
Hard Copy and
presentation
 On all imaging platforms, digitized
picture files are in standard formats and can be converted easily to other formats; data
can be exported to other computer systems or reproduced on a variety of hard copy devices.
Adobe Photoshop CS is most widely used for figure preparation and we are happy to
assist. A Fujix Pictrography 3000 color printer makes continuous tone output at 400
PPI indistinguishable from real photographs. Standard laser printing can be
used for draft grayscale as well as for crisp graphs and text. Both still images and moving sequences can be prepared for web
presentation. The AIF maintains in its inventory tools for video; however, use is by
special appointment as video is being phased out. On all imaging platforms, digitized
picture files are in standard formats and can be converted easily to other formats; data
can be exported to other computer systems or reproduced on a variety of hard copy devices.
Adobe Photoshop CS is most widely used for figure preparation and we are happy to
assist. A Fujix Pictrography 3000 color printer makes continuous tone output at 400
PPI indistinguishable from real photographs. Standard laser printing can be
used for draft grayscale as well as for crisp graphs and text. Both still images and moving sequences can be prepared for web
presentation. The AIF maintains in its inventory tools for video; however, use is by
special appointment as video is being phased out.
Computer support
Most routine image analyses run on
Windows XP PCs. A Mac G4 may be available by special request. Cryo EM
technologies are being developed on Linux PCs. The AIF staff are happy to assist
with any software but specialize in ImageJ, I.P. Lab, Lasersharp 2000, LCS (Leica
confocal), Microsoft Excel, Adobe Photoshop, VOXX and the 3D/4D portion of Volocity.
Windows XP serves all administrative needs. Researchers may transfer data to their
own computers via ALNET, by burning DVDs/CDs or by supplying their own Firewire or USB
hard drives or memory keys.
Networking
Most computers are networked to ALNET
to send data over the Internet.
Equipment availability
All equipment is available on a first come first served basis and at an hourly rate billed per quarter hour;
routine light microscopes are available at a yearly fee. Investigators may use the
equipment unassisted or they may have their work performed for them.
|